Pipeline
Phase II clinical study pipeline
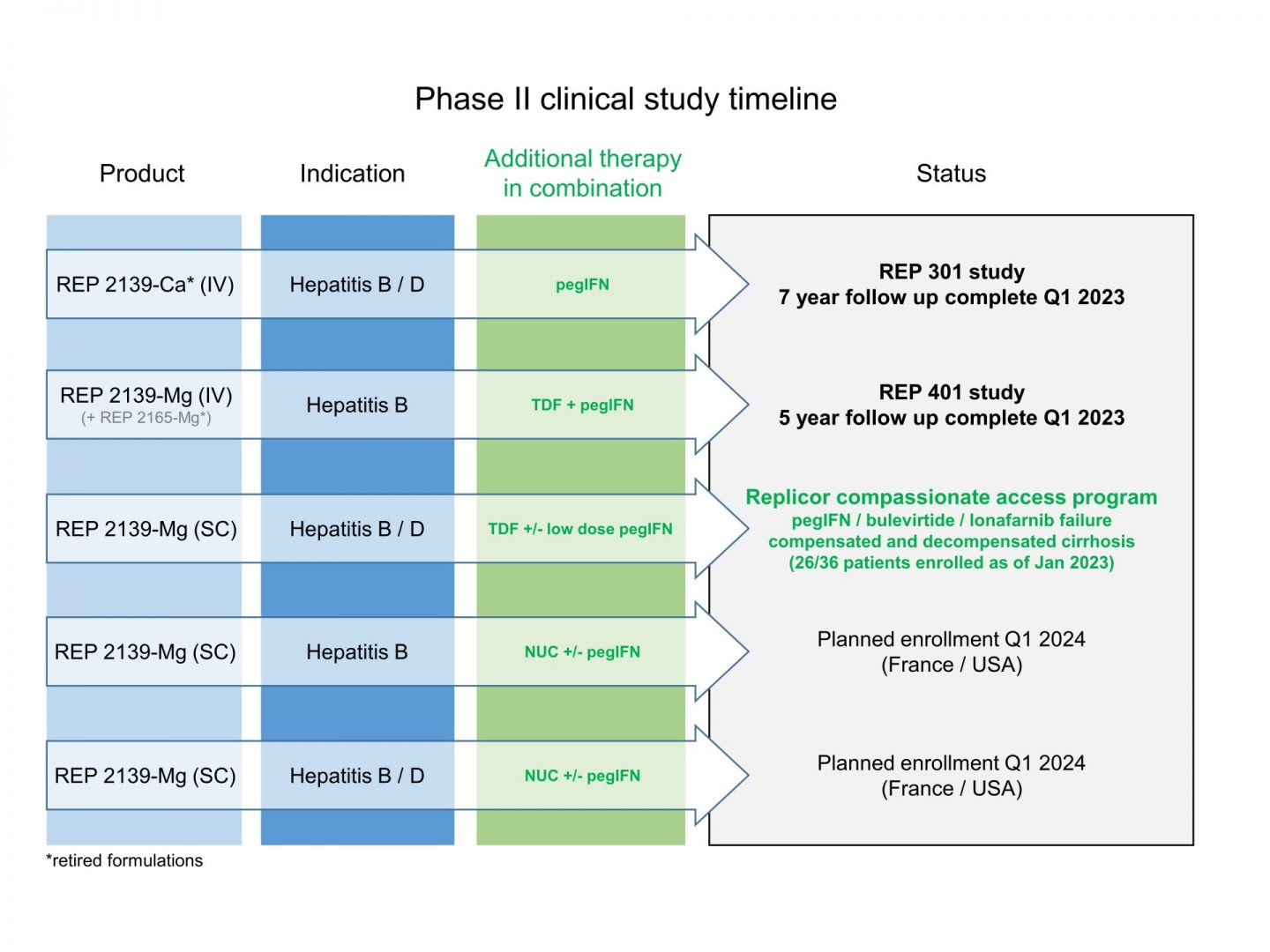
The development of an effective treatment for HBV and HBV / HDV infection will require a combination treatment. Initial phase II trials in Asia (see here ) evaluated dosing and combination regimens of early NAP formulations in HBeAg positive patients with maternal transfer (the most difficult patients in which to achieve functional cure).
Interim phase II trials (REP 301 see here and REP 401 see here) validated the activity of these regimens with combination therapy in HBeAg negative patients with or without HDV co-infection. These also led to the selection of REP 2139-Mg as the primary NAP candidate formulation for continued development for approval.
The current compassionate access program (see here) is currently underway and is successfully demonstrating the safety and efficacy of REP 2139-Mg by a convenient once weekly subcutaneous injection in patients with very advanced liver disease.
Two phase IIA studies are planned in patients with HBV and HBV / HDV co-infection which are expected to lead to accelerated phase IIB studies.
Replicor’s current clinical pipeline is as follows:
Replicor’s current clinical pipeline: