Hepatitis D
Hepatitis D, also called hepatitis delta, affects 15 to 20 million patients worldwide and is the deadliest of all forms of hepatitis. There is currently no approved treatment for this infection. Replicor’s lead NAP compound, REP 2139, is the first and only agent shown to be effective against both HBV and HDV infection in clinical trials.
What is Hepatitis D?
The hepatitis D (delta) virus (HDV) is a virus which can only replicate in the liver of patients who already have
HBV infection (please see here). This is because HDV does not contain the genes for producing a surface
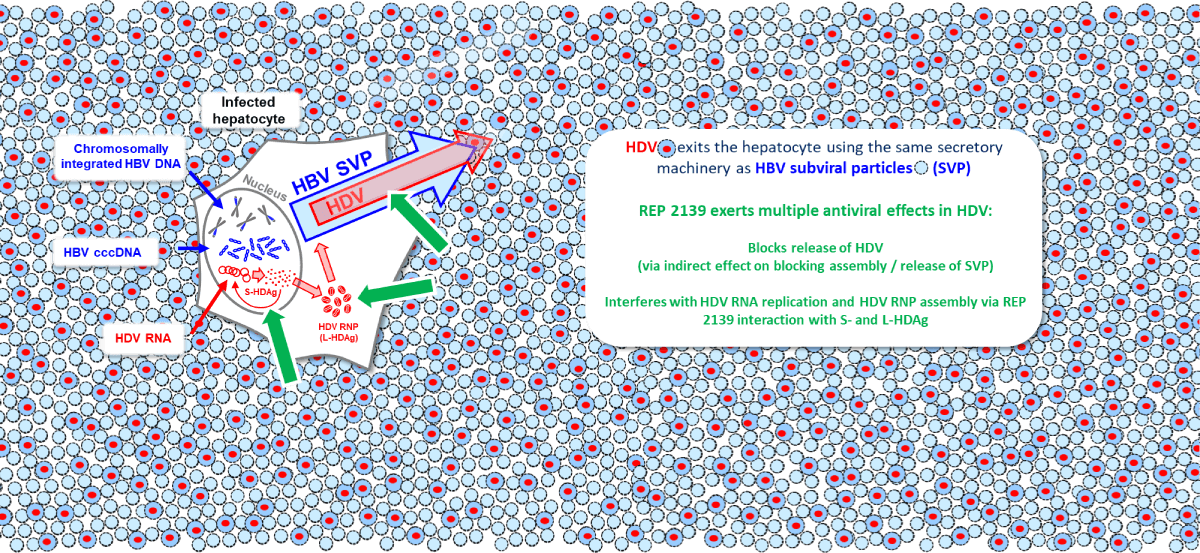
envelope protein and instead uses the surface antigen protein (HBsAg) produced either from cccDNA or
integrated HBV DNA. The process of the final assembly and secretion of HDV is identical to that of subviral
particles of HBV.
Current estimates are that HDV co-infection is present in 15-20 million people worldwide with HBV infection,
but recent new surveillance efforts indicate that this number underestimates the true prevalence of coinfections. This infection represents an urgent unmet medical need as, the progression of liver dysfunction to
fibrosis, cirrhosis and potentially hepatocellular carcinoma (liver cancer) is more rapid when HDV infection is
present than with any other form of viral hepatitis. The rapid evolution of liver disease during HDV co-infection
results in 80% of untreated patients developing cirrhosis within 10 years of becoming infected.
Limitations of approved therapy for HDV co-infection
The current goal of HDV therapy is to achieve functional cure of HDV, where HDV RNA remains undetectable
and liver function normal in the absence of therapy. Several different therapeutic approaches have been
attempted to treat HDV co-infection, the most successful being interferon, which can control HDV infection
during long term therapy (2 years) in up to 50% of patients. However, many of these patients relapse after
interferon therapy is withdrawn. Other experimental therapies which interfere with the maturation of the HDV
ribonucleoprotein particle (HDAg) have met with limited success in controlling HDV directly or in combination
with pegylated interferons and have little or no effect on HBsAg without which no significant immune control
of HBV or HDV infection can be expected. Myrcludex B (bulvertide) was recently approved as a daily
subcutaneous injection for the treatment of HDV infection in some European countries, but this agent has no
effect on HBsAg and as such, cannot establish functional cure of HBV. As such, therapy with this agent is
potentially life long.
NAPs directly target both HBV and HDV infections
REP 2139 effectively blocks the assembly and release of HBV SVPs (see the section on HBV), which use the
same host processes as HDV for secretion. Additionally, REP 2139 has a potent and direct antiviral effect
against HDV replication which is distinct from its effects on SVP assembly and release. This direct effect
against HDV replication appears to be mediated by the interaction of REP 2139 with the small and large forms
of the hepatitis delta antigen (See the section on HBV), which self assemble during HDV replication by the
interaction of exposed hydrophobic surfaces. These surfaces are efficiently targeted by REP 2139 and likely
result in inhibition of multiple steps in the HDV replication cycle. REP 2139 is unique in its ability to
simultaneously target HBsAg replenishment and multiple steps in the HDV lifecycle.
REP 2139 has achieved a breakthrough result in HBV / HDV co-infection.
In Replicor’s first clinical study, a limited exposure to REP 2139 therapy (30 weeks) with overlapping therapy
with pegylated interferon achieved clearance of both HBsAg and HDV RNA (the marker for HDV viremia in
the blood). As observed in HBV infection, these effects were accompanied by HBsAg seroconversion and
strong host mediated transaminase flares signalling the clearance of HBV infected hepatocytes.
Importantly, after 3.5 years of treatment free follow-up with this suboptimal regimen, 7/11 participants
completing therapy maintained functional cure of HDV, with all participants establishing control of their HDV
infection and 4 additionally maintaining functional cure of HBV. This potent HBV and HDV response is unique
amongst all agents currently in development or approved for HDV infection. Longer exposures to REP 2139
in combination with immunotherapy and NUCs is expected to significantly increase these response rates.