Press Release
Replicor successfully confirms NAPs target Hepatitis B subviral particles
MONTREAL, April 4th, 2022 – Replicor Inc., announced the successful confirmation of the targeting of Hepatitis B subviral particles (SVP) by NAPs in its REP 301 and REP 401 clinical studies.
Clearance of SVP during therapy is required to achieve functional cure of HBV; circulating HBsAg is comprised of >99.99% non-infectious SVP, which prevents immune control of infection. Efficient clearance of SVP in the clinic can be confirmed by the selective decline of the small isoform of HBsAg (S-HBsAg), which is uniquely enriched in these particles.
In a new analysis published in Hepatology Communications (here), performed in collaboration with Abbott Diagnostics, rapid clearance of HBsAg during NAP-based therapy of HBV monoinfection (REP 401 study) or HBV / HDV coinfection (REP 301 study) was accompanied by selective clearance of S-HBsAg. These results confirm the efficient clearance of SVP by NAPs in human infection, consistent with the selective inhibition of the assembly and secretion of SVP by NAPs observed in previous in vitro mechanistic studies here and here.
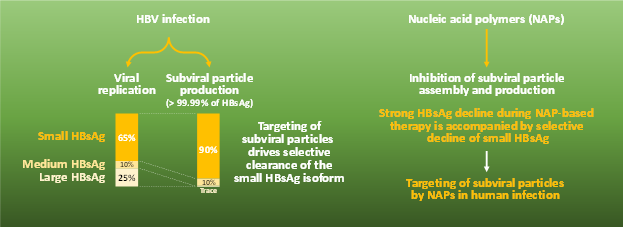
Dr. Andrew Vaillant, CSO, Replicor commented, “Efficient clearance of subviral particles is a critical step in achieving functional cure of HBV. No other antiviral therapy in development (including NUCs, CAMs or RNAi) has this effect, which underlies the unique ability of NAP-based therapy to achieve high rates of functional cure of HBV.”
About Replicor
Replicor is a privately held biopharmaceutical company with the most advanced animal and human clinical data in the development of the cure for HBV and HDV. The company is dedicated to accelerating the development of an effective treatment for patients with HBV and HBV/HDV co-infection. For further information about Replicor please visit our website at www.replicor.com.
